
17/11/2025
In Vietnam, the aquaculture and seafood processing industry plays a vital role in economic growth and exports but also generates large amounts of solid waste and wastewater, putting pressure on coastal environments. Oysters and other mollusks are increasingly farmed, producing substantial shell waste each year. While part of this is reused in animal feed or as artificial reefs, most oyster shells are directly discarded, causing pollution and resource waste. In the context of a shift toward sustainable and environmentally friendly consumption, recycling oyster shells is highly practical, helping to reduce pollution while adding economic value. With calcium carbonate (CaCO₃) as their main component, oyster shells hold strong potential for application in seafood processing wastewater treatment. This contributes to mitigating coastal environmental pollution while also promoting a sustainable pathway for the fisheries industry.
1. INTRODUCTION
The aquaculture and seafood processing industry makes a significant contribution to the national economy and foreign exchange earnings; however, it also generates environmental pollution due to the large volumes of solid waste and wastewater released during production. According to the Vietnam Environment Administration (2011), the processing of catfish produces 5 - 7 m3 of wastewater per ton of product, with pollutant concentrations exceeding permissible standards. If left untreated, these discharges can exert adverse impacts on the environment (Nguyen Vo Chau Ngan et al., 2015).
The degree of pollution in wastewater generated from seafood processing varies considerably, depending on the type of raw materials (e.g., shrimp, fish, squid, octopus, crab, clams, mussels), as well as seasonal fluctuations and even daily production activities. In particular, processing lines are often characterized by high concentrations of pollutants, with pH ranging from 6.5 to 7.0, suspended solids (SS) from 500 to 1,200 mg/L, chemical oxygen demand (COD) from 800 to 2,500 mgO2/L, biochemical oxygen demand over 5 days (BOD5) from 500 to 1,500 mgO₂/L, total nitrogen from 100 to 300 mg/L, total phosphorus from 50 to 100 mg/L, and oil and grease from 250 to 830 mg/L (Le Hoang Viet et al., 2014). These values indicate that seafood processing wastewater is rich in organic matter and highly biodegradable, as reflected by the BOD/COD ratio, which ranges from 0.6 to 0.9 (Nguyen Trung Viet et al., 2011; Pham Thi Phuong Trinh et al., 2016). Conventional wastewater treatment methods, such as aerobic and anaerobic processes, microbial applications, or chemical treatments, typically require high capital and operational costs, while also generating secondary sludge that poses additional challenges for environmental management.
Oysters in particular, and mollusks in general, are widely farmed in coastal regions. Globally, hundreds of tons of shell waste from mollusks, including oyster shells, are generated annually, posing a significant environmental burden (W.H. Park et al., 2008). In Vietnam, the average oyster production in three coastal provinces - Quang Ninh, Hai Phong, and Thanh Hoa - reaches approximately 10,000 - 12,000 tons per year. Estimated production in other provinces is as follows: Nghe An, 2,000 tons/year; Thua Thien - Hue, 2,500 tons/year; Phu Yen, 1,800 tons/year; and Ba Ria - Vung Tau, 2,000 tons/year, while most remaining coastal provinces produce 500 - 1,500 tons annually. Overall, Vietnam’s oyster production amounts to 30,000 - 35,000 tons per year, corresponding to an estimated 25,500 - 29,700 tons of oyster shell waste (Nguyen Xuan Thi et al., 2011; Fisheries Encyclopedia, 2007; Nguyen Xuan Thi, 2019).
Oyster shells represent an abundant waste source from aquaculture and seafood processing. Their reuse not only reduces the burden of solid waste management but also offers potential as an effective adsorbent material. Numerous studies have demonstrated the capacity of oyster shells to remove heavy metals; however, applications for treating organic matter, nutrients, and microorganisms in seafood processing wastewater remain limited. Compared with other mollusks, oysters are produced in large quantities, and their shells consist primarily of CaCO3, which can be readily transformed into CaO and other active derivatives, highlighting their potential for the remediation of diverse pollutants. Recycling oyster shells could therefore mitigate coastal environmental pollution while providing a sustainable pathway for the aquaculture industry. At present, however, their utilization has mainly been restricted to animal feed additives and artificial reef construction, with limited effectiveness. Consequently, research on oyster shell recycling - particularly the transformation mechanisms of CaCO3 under different conditions for wastewater treatment in seafood processing - holds significant practical value and promising prospects in Vietnam.
2. LITERATURE REVIEW
2.1. International Research
Worldwide, several studies have investigated the reuse of oyster shell powder as an adsorbent material for environmental pollution control (Nguyen Xuan Thai, 2019). This potential is attributed to the high CaCO3 content in oyster shells, which can be converted into CaO after thermal treatment, enabling effective wastewater treatment. A study by Darioush Alidoust and colleagues in Japan reported that the chemical composition of oyster shells consisted of 97.21% CaO, 0.80% Na2O, 0.61% SO3, 0.35% MgO, 0.39% SiO2, 0.24% SrO, 0.11% P2O5, 0.07% K2O, 0.11% Al2O3, 0.09% Fe2O3, 0.01% TiO2, and 0.01% MnO (Darioush Alidoust, 2015).
In the study conducted by Asaad F. Hassan and Radim Hrdina, oyster shells were calcined at 9000C to convert CaCO3 into CaO. The resulting CaO was finely ground and reacted with HNO3 to synthesize Ca(NO3)2, while a 0.5 M H2PO4 solution was added to maintain the pH within the range of 10 - 11. The mixture was then dried at 1100C and subsequently treated in a microwave furnace at 8000C, yielding nano - sized Ca(NO3)2. This nanomaterial was applied for mercury ion adsorption, and the results demonstrated high adsorption efficiency as well as desorption capacity, confirming its potential for heavy metal pollution treatment (AsaadF. Hassana et al., 2018; Nguyen Xuan Thai, 2019).
Darioush Alidoust and colleagues treated oyster shells collected from restaurants in Japan by first brushing off the attached algae, followed by crushing and calcination at different temperatures (4500C, 6500C, 7500C, 8000C, 9000C) for 2 hours. The calcined materials were then evaluated for their cadmium ion (Cd2+) adsorption capacity. The results indicated that thermally treated oyster shells exhibited significantly enhanced Cd2+ adsorption efficiency and capacity compared to the raw material (Darioush Alidoust, 2015; Nguyen Xuan Thai, 2019).
In the study by Hsing Yuan Yen and colleagues, calcined oyster shells demonstrated a strong ability to adsorb nickel ions (Ni2+). Oyster shells calcined at 9000C exhibited a porous structure with numerous micropores on the surface. The adsorption efficiency of Ni2+ using shells calcined at 9000C was significantly higher than that of shells calcined at 6000C. Specifically, the adsorption efficiency at 6000C was 48.3%, while at 9000C it reached 99.9% under conditions of pH 10 and an adsorption temperature of 600C (Hsing Yuan Yen et al., 2015). Similarly, Liwei Fan and colleagues developed a composite material from oyster shells and nanoscale iron for arsenic (As3+) adsorption. The material achieved an adsorption efficiency of 96.5% under conditions of pH 6.8, temperature 200C, an initial As3+ concentration of 1.8 ppm, and an adsorption time of 24 hours (Liwei Fan et al., 2015).
Based on the analysis of the adsorption properties of oyster shells, Huang, Mian-Li and colleagues investigated the treatment of phosphorus-containing wastewater using oyster shells and examined the effect of temperature on phosphorus removal efficiency. X-ray diffraction (XRD) analysis was employed to identify crystalline phases, revealing that CaCO3 was the principal component of oyster shells. At a pretreatment temperature of 8000C, part of the CaCO3 was decomposed into CaO, and the amount of CaO increased with higher temperatures. This thermal pretreatment activated calcium species, thereby significantly enhancing phosphorus adsorption from wastewater (Huang et al., 2010).
In South Korea, Jae-Hoon Huh and colleagues investigated the use of oyster shell powder to improve the water quality of lakes by controlling algal blooms. Waste oyster shells were calcined at 10000C for 1 hour in air, converting raw oyster shell powder into calcium oxide powder, which effectively reacted with phosphorus and nitrogen to remove algal blooms from eutrophic wastewater. The application of oyster shell powder resulted in reductions of 97% in total phosphorus, 91% in total nitrogen, and up to 51% in chemical oxygen demand (COD), compared with the total pollutant load of untreated algal solutions (Jae-Hoon Huh et al., 2016).
The antibacterial potential of nano- and micro-sized thermally treated oyster shell powders was investigated by Watanabe and colleagues using two bacterial strains: E. coli and B. subtilis. Nano-sized particles derived from oyster shells were prepared using a wet milling method followed by heat treatment (HSS), and their antibacterial activity was compared with that of micro-sized particles against both bacterial cells and spores. The sporicidal activity of the nano-sized particles was markedly higher than that of the micro-sized particles, with HSS-derived nanoparticles demonstrating the ability to inactivate Bacillus subtilis spores. Specifically, the number of B. subtilis spores decreased nearly threefold after 30 minutes of exposure to HSS nanoparticles at a concentration of 5 mg/mL and 600C (Watanabe T. et al., 2014; Nguyen Xuan Thi, 2019).
The study by Zheng Liu et al. employed pyrolyzed oyster shell (POS) to remove Cu(II) from aqueous solution. The Taguchi method was applied to optimize process conditions and identify the key influencing factors. Results indicated that pyrolysis temperature and reaction time were the two determining factors, with Cu(II) removal efficiency reaching 99.82% at 250C, 9000C, 100 mg/L, 0.2 g POS, and 30 minutes. Adsorption kinetics followed the pseudo-second-order model, while the Langmuir isotherm accurately described the experimental data, with a maximum adsorption capacity of 1093 mg/g at 9000C (Zheng Liu et al., 2024).
Oyster shell (OS) is commonly used to mitigate acidification during methane production but is limited by its low electron transfer efficiency. This study developed a magnetite - oyster shell composite (MOC) with different Fe3O4 contents to simultaneously buffer pH and enhance electron transfer. The results showed that MOC exhibited superior redox activity, conductivity, and pH buffering capacity compared to OS and Fe3O4 alone. MOC15% (10 g/L) achieved the best performance, increasing methane yield by 9.7 times and improving pH by 44% compared to the control. The enhanced efficiency was attributed to its dual role in mitigating acid stress and promoting direct interspecies electron transfer (DIET), as well as enriching microorganisms such as Methanothrix and Geobacter. This provides a sustainable and cost-effective solution to enhance biomethane recovery from high-COD, carbohydrate-rich wastewater (Fangyuan Feng et al., 2025).
Studies by Kikuo et al. (2000), Achanai Buasri et al. (2013), Jingyu et al. (2018), and Ramakrishna Chilakala et al. (2019) also demonstrated that treated oyster shells can adsorb or remove various wastewater contaminants such as coliform, E. coli, and phosphates.
Thus, worldwide, numerous studies have focused on the use of oyster shells for the removal of heavy metals from wastewater. However, research on their application for the treatment of organic matter, nutrients, and coliforms remains limited.
2.2. In Vietnam
In relation to the present research topic, several studies have been conducted in Vietnam as follows:
Nguyen Xuan Thai successfully treated and modified oyster shell powder samples collected from the two coastal provinces of Quang Ninh and Phu Yen. The adsorption capacity of these treated samples for the heavy metal ion Cr6+ was subsequently evaluated. The results indicated that the morphology and structure of the treated oyster shells differed significantly from those of the untreated samples, with the treated shells exhibiting a porous structure and numerous micropores on their surfaces (Nguyen Xuan Thai, 2019).
Ngo Thuy Diem Trang and colleagues investigated the effect of calcination temperature on the phosphorus adsorption capacity of blood cockle (Anadara granosa) shell powder. The study evaluated three temperature levels (5500C, 7500C, and 9500C) using shell particles of ≤2.0 mm. Phosphorus adsorption experiments were conducted over 24 hours at an initial concentration of 20 mg PO43-/L. The results demonstrated that thermally treated shells exhibited a higher phosphorus adsorption capacity compared with untreated shells; however, effective performance required calcination above 7500C. At 9500C, the phosphorus adsorption efficiency reached 99.2% (Ngo Thuy Diem Trang et al., 2017).
Tran Do Mai Trang investigated the treatment of freshwater mussel shell powder and evaluated its adsorption capacity for methylene blue dye in aqueous solution. The results showed that pretreated mussel shell powder (referred to as "initial shell powder") exhibited good adsorption capacity for methylene blue, with an efficiency of approximately 80%. The initial shell powder also demonstrated moderate adsorption capacity for Cr(VI) ions, with an efficiency of around 50%. The optimal conditions for Cr(VI) adsorption in aqueous solution were identified as 0.5 g of adsorbent, pH 7, temperature 300C, an initial methylene blue concentration of 10 ppm, and a contact time of 70 minutes (Tran Do Mai Trang, 2022).
Nguyen Xuan Thi and colleagues optimized the calcination process of oyster shells. The optimization results identified the suitable conditions for shell calcination as a temperature of 8500C, a duration of 92 minutes, and shell lengths of 4.0 - 7.0 cm, yielding a CaO recovery rate of 55.75% relative to the raw shells. X-ray diffraction analysis revealed that under these optimized conditions, CaCO3 crystals were no longer present; instead, characteristic peaks of CaO appeared, while no peaks of CaCO3 were detected. This confirmed that CaCO3 in the oyster shells was completely decomposed during calcination (Nguyen Xuan Thi et al., 2018).
Duong Thi Minh Hoa and colleagues investigated the modification of clam shells for the removal of Pb from contaminated water. The study prepared two types of materials: cleaned clam shell powder dried for 12 hours (control) and thermally modified clam shell powders calcined at 4000C, 5000C, 6000C, 7000C, 8000C, 9000C, and 10000C, each exhibiting distinct properties. At 10000C, CaCO3 was completely decomposed into CaO, resulting in the highest adsorption performance. The modified clam shell at 10000C achieved a removal efficiency of 99.67% with an adsorption capacity of 2.990 (Duong Thi Minh Hoa et al., 2022).
Thus, in Vietnam, studies on the application of oyster shells for wastewater pollution treatment remain limited and have primarily focused on individual pollutants. The treatment capacity of oyster shell powder for specific types of industrial wastewater, as well as the optimal experimental conditions required to achieve maximum efficiency, has not yet been comprehensively evaluated.
3. PROPOSED RESEARCH CONTENT
3.1. Research Objectives
To evaluate the removal capacity of treated oyster shell powder for organic matter, nutrients, and coliforms from seafood processing wastewater.
3.2. Investigation of modified oyster shells for the treatment of pollutants (organic matter, nutrients, and coliforms) in wastewater from the seafood processing industry
Collection and preliminary treatment of discarded oyster shells.
Calcination of oyster shells at different temperatures: 7500C, 8000C, 8500C, 9000C, 10000C.
Grinding of calcined oyster shells to obtain powder, followed by storage in separate containers for experimental preparation.
3.3. Experimental study on the pollutant removal capacity (organic matter, nutrients, and coliforms) of oyster shell powder in seafood processing wastewater
Evaluation of the treatment efficiency of oyster shell powder under different experimental conditions. If the removal efficiency is satisfactory, the experiments may be repeated with a lower dosage of powder; conversely, the dosage may be increased if the efficiency remains low.
4. PROPOSED RESEARCH METHODS AND TECHNIQUES
4.1. Research methodology
a) Literature review methodology: Collection and utilization of existing materials from research subjects or relevant articles and reports published in specialized journals.
b) Survey and sampling method for seafood processing wastewater: Sampling is conducted in accordance with Circular No. 10/2021/TT-BTNMT on environmental monitoring techniques and the management of environmental quality monitoring information and data. After sampling, the handling and preservation of samples for laboratory analysis are carried out following the guidelines of Standard Methods for the Examination of Water and Wastewater, 24th edition (APHA, 2023).
c) Procedure for the treatment and modification of oyster shells (Nguyen Xuan Thai, 2019)
Oyster shells were collected, washed, and air-dried, then soaked in a NaClO (Javel water) solution for 24 hours to remove algae, sand, impurities, and residual organic matter. Afterward, the shells were rinsed with distilled water and dried to constant weight before being ball-milled with a NaOH/NaClO solution (shells/solution ratio of 8/40 g/mL, NaOH/NaClO ratio of 80/20) for 24 hours. The resulting oyster shell powder was again washed with distilled water and oven-dried in a natural convection air oven at 1000C until constant weight. Calcination was then carried out at different temperatures (7500C, 8000C, 8500C, 9000C, and 10000C) for 2 hours. After cooling, the calcined oyster shell powder was stored in sealed polyethylene (PE) bags.
Modification of pretreated oyster shell powder with EDTA was performed as follows: 0.3 g of EDTA was accurately weighed and dissolved in 50 mL of distilled water using a magnetic stirrer at 600C. Subsequently, 3 g of oyster shell powder was added to the solution and stirring was continued for 2 hours. Finally, the oyster shell powder was washed with distilled water, and the solid fraction was collected by filtration. The powder was then oven-dried at 1000C until constant weight.
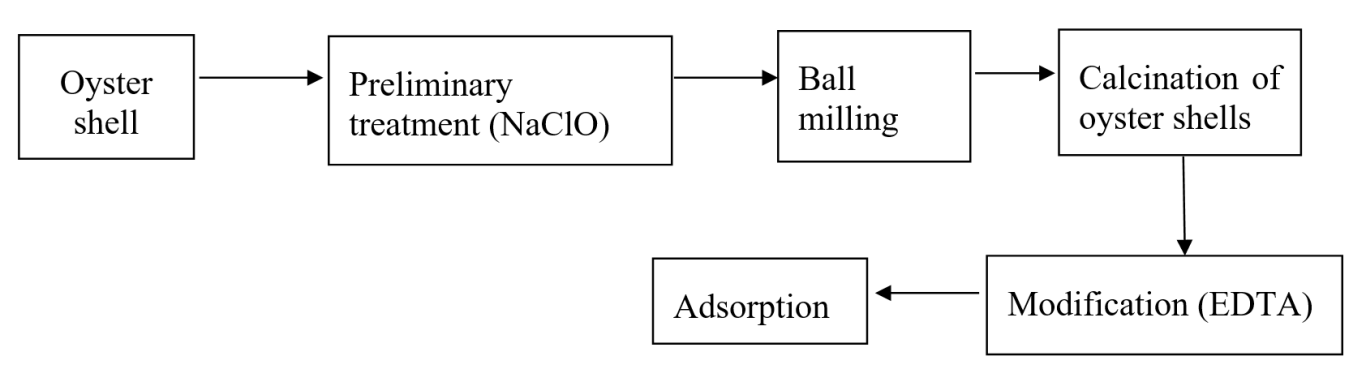
Figure 1. Process of treatment and modification of oyster shells
d) Experimental design for evaluating the pollutant removal capacity (organic matter, nutrients, and coliforms) of oyster shell powder in seafood processing wastewater.
- Collection of seafood processing wastewater samples and analysis of pollutant concentrations (BOD5, COD, TSS, NH4+, PO43-, coliform) in the initial wastewater.
- Division of wastewater into separate containers (V liters) with the addition of treated oyster shell powder, followed by experiments under different conditions:
+ Different dosages of oyster shell powder used in the experiments, for example: 20 g/L; 50g/L; 100 g/L.
+ Different experimental durations: 1 day, 2 days, and 5 days.
- Collection of wastewater samples after the experiments and evaluation of pollutant concentrations (BOD5, COD, TSS, NH4+, PO43-, coliform) in the effluent.
Note: Measure the pH of the influent and effluent wastewater in the experiments. All experiments are conducted using a magnetic stirrer to homogenize the test samples.
4.2. Analytical techniques employed
a) Techniques for measuring parameters in the field and in the laboratory
The pH of the wastewater is measured using a pH meter with an accuracy of 0.01 pH units. The wastewater temperature is displayed on the pH meters equipped with a temperature probe.
b) Techniques for analyzing wastewater samples in the laboratory
TSS analysis method: The TSS content in wastewater is determined by filtering an accurately measured volume of the sample through a filter membrane. The membrane containing the retained solids is rinsed with distilled water to remove salts, oven-dried at 1050C to constant weight, cooled, and weighed.
BOD5 analysis method: The sample is incubated at 200C for a fixed period of five days in the dark, in completely filled and tightly sealed bottles. The dissolved oxygen concentration is measured before and after incubation, and the amount of oxygen consumed per liter of sample is calculated.
COD analysis method: Potassium permanganate (KMnO4) is used to oxidize organic compounds in an alkaline medium. The amount of potassium permanganate consumed in the reaction is expressed as mg O2 per liter.
Phosphate (PO43-) analysis method: The determination of phosphate ions in water is based on their reaction with ammonium molybdate reagent in an acidic medium to form a yellow phosphomolybdate complex, H7[P(Mo2O7)6]. This complex, when reduced with stannous chloride (SnCl2), produces a molybdenum blue complex. The absorbance of the solution is then measured at a wavelength of 690 nm using a UV-VIS spectrophotometer.
Ammonium (NH4+) analysis method: Ammonium is determined according to SMEWW 4500-NH3 F using the colorimetric method on a DR/3900 spectrophotometer (HACH, USA).
Coliform analysis method: The determination of coliform density in wastewater is carried out in accordance with TCVN 6187-2:1996 (Detection and enumeration of coliform bacteria, thermotolerant coliform bacteria, and presumptive Escherichia coli).
c, Data processing method
- Adsorbed mass and percentage of adsorbed substance (Tran Do Mai Trang, 2022).
The mass of substance adsorbed per gram of adsorbent, Q (mg/g), is calculated using the following formula:
(1)
Where Q is the amount of substance adsorbed per gram of adsorbent at equilibrium (mg/g); C0 and Ce are the initial and equilibrium concentrations of the adsorbate in solution (mg/L), respectively; V is the solution volume (L); and W is the mass of the adsorbent (g).
The percentage of substance adsorbed is calculated as follows:
(2)
- Investigation and development of adsorption isotherm equations (Tran Do Mai Trang, 2022).
In this study, the Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherm models were selected to investigate the adsorption process in the solid–liquid system (oyster shell powder - wastewater samples containing BOD5, COD, TSS, NH4+, PO43-, and coliform). The Langmuir isotherm equation describes the adsorption process in solution by a solid adsorbent as follows:
(3)
Where Qe is the amount of substance adsorbed per gram of adsorbent at equilibrium (mol/g), Ce is the equilibrium concentration of the solute (mol/L), and Q0 and kL are Langmuir constants, with Q0 representing the maximum monolayer adsorption capacity and kL representing the binding energy or affinity parameter of the adsorption system.
The Freundlich isotherm equation for the above system is expressed as follows:
(4)
Where kF is the Freundlich isotherm constant (L1/n mg(1−1⁄n)g-1) and 1/nF is the Freundlich exponent.
The Temkin isotherm equation for the above system is expressed as follows:
Qe = B.lnAT + B.ln.Ce với B = RT/bT (5)
Where AT is the Temkin isotherm equilibrium binding constant (L/g), bT is the Temkin isotherm constant, R is the gas constant (R = 8.314 J/mol/K), and T = 298 K.
The Dubinin - Radushkevich (D - R) isotherm equation for the above system is expressed as follows:
(6)
Where Qs is the theoretical saturation capacity of the isotherm (mg/g) and Kad is the Dubinin - Radushkevich isotherm constant (mol2/kJ2).
- Investigation of adsorption kinetics (Tran Do Mai Trang, 2022).
In this study, the first - order, pseudo - first - order, second - order, and pseudo - second - order adsorption kinetic models were selected to investigate the adsorption kinetics of wastewater samples (BOD5, COD, TSS, NH4+, PO43-, coliform) using oyster shell powder.
First-order adsorption kinetic model:
lnCt = lnC0 –k1t (7)
Pseudo-first-order adsorption kinetic model:
ln (Q0 - Qt) = lnQ0 – ks1t (8)
Second-order adsorption kinetic model:
(9)
Pseudo-second-order adsorption kinetic model:
(10)
Where C0 and Ct are the solute concentrations at the initial time and at time t (mg/L); Q0 is the maximum monolayer adsorption capacity (mg/g); Qt and Qe are the amounts of substance adsorbed per gram of adsorbent at time t and at equilibrium (mg/g); and k1, ks1, k2, and k are the rate constants for the first-order, pseudo-first-order, second-order, and pseudo-second-order kinetic models, respectively.
5. CONCLUSION
The overview indicates that, globally, numerous studies have focused on using oyster shells for the removal of heavy metals from wastewater, whereas research on the treatment of organic matter, nutrients, or microorganisms remains limited. In Vietnam, this research area is still at an early stage, primarily addressing the removal of individual pollutants, without a comprehensive assessment of the potential application of oyster shells for various types of industrial wastewater or the optimization of experimental conditions. Given that seafood processing wastewater is generated in large volumes with high pollutant concentrations (COD, BOD, nitrogen, phosphorus, oils and fats, etc.), posing significant treatment challenges due to high investment and operational costs, the utilization of waste oyster shells as a recycled material for wastewater treatment offers both environmental benefits and practical prospects. Therefore, in-depth studies are needed to explore the potential of oyster shells in treating seafood processing wastewater.
Acknowledgments: This research is the outcome of project conducted at the Vietnam Academy of Science and Technology (VAST07.05/24-25). The authors express their gratitude to the Vietnam Academy of Science and Technology for financial support in conducting the research.
Lê Văn Nam*
Graduate University of Science and Technology - Vietnam Academy of Science and Technology
Cao Thị Thu Trang, Nguyễn Thị Mai Lưu, Nguyễn Thị Thu Hà, Nguyễn Thị Kim Anh, Trần Anh Tú, Bùi Thị Mai Huyền, Lê Văn Nam
Institute of Science and Technology for Energy and Environment - Vietnam Academy of Science and Technology
Nguyễn Hồng Ngọc
Centrer for Hight Technology Research and Development - Viet Nam Academy of Sience and Technology
(Source: The article was published on the Environment Magazine by English No. III/2025)
REFERENCES
1. Achanai Buasri, Nattawut Chaiyut, Vorrada Loryuenyong, Phatsakon Worawanitchaphong, and Sarinthip Trongyong, 2013. Calcium Oxide Derived from Waste Shells of Mussel, Cockle, and Scallop as the Heterogeneous Catalyst for Biodiesel Production. Hindawi Publishing Corporation The Scientific World Journal Volume 2013, Article ID 460923, 7 pages, http://dx.doi.org/10.1155/2013/460923.
2. American Public Health Association, American Water Works Association, Water Environment Federation. Lipps WC, Braun - Howland EB, Baxter TE, eds. 2023. Standard Methods for the Examination of Water and Wastewater. 24th ed. Washington DC: APHA Press.
3. AsaadF. Hassana, RadimHrdina, 2018. Chitosan/nanohydroxyapatite composite based scallop shells as an efficient adsorbent for mercuric ions: Static and dynamic adsorption studies international Journal of Biological Macromolecules 109 (2018) 507 - 526.
4. Darioush Alidoust, 2015. Mechanism of cadmium biosorption from aqueous solutions using calcined oyster shells Journal of Environmental Management 150 (2015) 103-110.
5. Duong Thi Minh Hoa, Tran Thi Pha, Hang A Hong, Chu Thi Xuan Hao, Nguyen Van Giap, Nguyen Minh Tung, Ly Thanh Thien, Ma Thi Diem, Nguyen Thi Kieu Trang, Vuong Thi Thu Thao, Vang A Khai, Mang Thi May, 2022. Research on modified clam shell to treat Pb in polluted water. Scientific Journal of Tan Trao University, Vol 8. No.2_ June 2022, (in Vietnamese).
6. Encyclopedia of Fisheries, 2007. Estuarine oysters. Agricultural Publishing House, (in Vietnamese).
7. Fangyuan Feng, Yichao Wang, Jingyang Luo, Chunhui Zhao, Yongfang Zhang, Chuanshun Zhi, Hui Mu, 2025. Journal of Environmental Chemical Engineering, Volume 13, Issue 6, December 2025, 119289, https://doi.org/10.1016/j.jece.2025.119289.
8. Hsing Yuan Yen, Jun Yan Li, 2015. Process optimization for Ni(II) removal from wastewater by calcined oyster shell powders using Taguchi method Journal of Environmental Management 161 (2015) 344-349.
9. Huang, Mian-Li, Yu, Yan, Wu, Ren-ping, 2010. Researches on the Treatment of Phosphorous Wastewater with Oyster Shells. Chinese Journal of Structural Chemistry 29(12):1886-1892.
10. Jae-Hoon Huh, Young-Hoon Choi, Hyun-Jae Lee, Woo Jeong Choi, Chilakala Ramakrishna, Hyoung-Woo Lee, Shin-Haeng Lee, and Ji-Whan Ahn, 2016. The Use of Oyster Shell Powders for Water Quality Improvement of Lakes by Algal Blooms Removal. Journal of the Korean Ceramic Society Vol. 53, No. 1, pp. 1~6, 2016. http://dx.doi.org/10.4191/kcers.2016.53.1.1.
11. Jingyu Zhang, Hong Chen, TaoMu and Yi Pan, 2018. Research and Application of Shell Powder. IOP Conf. Series: Earth and Environmental Science 170 (2018) 032031 doi :10.1088/1755-1315/170/3/032031.
12. Kikuo Oikawa, Takashi Asada, Kazuo Yamamoto, Hiroyuki Wakabayashi, Manabu Sasaki, Makoto Sato, Junji Matsuda, 2000. Antibacterial Activity of Calcined Shell Calcium Prepared from Wild Surf Clam. Journal of Health Science, Volume 46 Issue 2, Pages 98-103.
13. Le Hoang Viet, Nguyen Vo Chau Ngan, 2014. Textbook of Wastewater Treatment Engineering. Can Tho University Publishing House, (in Vietnamese).
14. Liwei Fan, Shuili Zhang, 2015. Removal of arsenic from simulation wastewater using nano-iron/oyster shell composites Journal of Environmental Management 156 (2015) 109-114.
15. Ngo Thuy Diem Trang, Trieu Thi Thuy Vi, Le Nguyen Anh Duy, Tran Sy Nam, Le Anh Kha, Pham Viet Nu, 2017. Effect of thermal treatments on phosphorus adsorption capacity of cockle-shell powder. CTU Journal of Science, Volume 50, Part A (2017): 77-84, (in Vietnamese).
16. Nguyen Trung Viet, Tran Thi My Dieu, Huynh Ngoc Phuong Mai, 2011. Environmental engineering chemistry (part I). Science and Technics Publising House, (in Vietnamese).
17. Nguyen Vo Chau Ngan, Nguyen Thi Kim Ngan, Huynh Quoc Truong, Le Hoang Viet, 2015. Evaluating treatment efficient of fish processing wastewater by trickling filter tank with cocopeat medium and sawdust medium. CTU Journal of Science, Part A: Natural Sciences, Technology and Environment: 37 (2015): 51-62, (in Vietnamese).
18. Nguyen Xuan Thai, 2019. Master's thesis in chemistry: Research on the characteristics of oyster shell powder and the ability to adsorb some heavy metal ions. Graduate University of Science and Technology, Vietnam Academy of Science and Technology, (in Vietnamese).
19. Nguyen Xuan Thi, 2019. PhD thesis in food technology: Research on the technology of producing calcium carbonate from oyster shells as a food additive. Hanoi University of Science and Technology, (in Vietnamese).
20. Nguyen Xuan Thi, La The Vinh, Pham Thi Diem, 2018. Study on the optimization of oyster shell calcination, Journal of Agriculture and Rural Development, Issue 1, July 2018, (in Vietnamese).
21. Nguyen Xuan Thi, Nguyen Van Doan, Pham Thi Diem, Nguyen Van Thong, et al., 2011. Comprehensive report on the research of pharmaceutical - grade calcium carbonate production from oyster shells. Ministry of Science and Technology, pp. 29 - 73, 144 - 160, (in Vietnamese).
22. Pham Thi Phuong Trinh, Nguyen Thi Hong Tham, Nguyen Thanh Quang, Nguyen Thi Thuy Trang, Dao Minh Trung, 2016. Application of the biological flocculants to processing fish wastewater. Thu Dau Mot University Journal of Science, No. 2 (27) - 2016, (in Vietnamese).
23. Ramakrishna Chilakala, Chottitisupawong Thannaree, Eunsoo Justin Shin, Thriveni Thenepalli and Ji Whan Ahn, 2019. Sustainable Solutions for Oyster Shell Waste Recycling in Thailand and the Philippines. MDPI, Recycling 2019, 4, 35; doi:10.3390/recycling4030035.
24. Tran Do Mai Trang, 2022. Master’s thesis in Chemistry: Study on the treatment of mussel shell powder and evaluation of its adsorption capacity for methylene blue in aqueous solution. Graduate University of Science and Technology, Vietnam Academy of Science and Technology, (in Vietnamese).
25. W.H. Park, C. Polprasert, 2008. Roles of oyster shells in an integrated constructed wetland system designed for P removal ecological engineering 34 (2008) 50 - 56.
26. Watanabe T, Fujimoto R, Sawai J, Kikuchi M, Yahata S, Satoh S, 2014. Antibacterial characteristics of heated scallop-shell nano-particles. Biocontrol Science, Vol 19, No2, 93-97 pages.
27. Zheng Liu, Shujian Wu, Rongmei Mou, 2024. Efficient removal of Cu(II) from aqueous solution using pyrolyzed oyster shells by Taguchi method, Biomass Conversion and Biorefinery (2024) 14:1175–1186, https://doi.org/10.1007/s13399-023-03884-9.