
21/12/2020
Recently, the quickly increasing of population, and variety of anthropogenic activities such as urbanization, industrialzation put the freshwater ecosystem in the risks of contamination with xenobiotics released by anthropogenic activities. Among pollutants, heavy metals are considered as the most toxic elements to aquatic living organisms and human health. In Southeastern region of Việt Nam, Sài Gòn River not only provides drinking water for million people, but also supports biodiversity of local freshwater ecosystem. The aim of this study is to assess the sensibility of freshwater microalgae Scenedesmus sp. and water flea Daphnia carinata, two fresh water species from Việt Nam to chromium (Cr). After physical and chemical characterization, field water samples from upstream of Sài Gon River were used as dilution water in toxicity tests. With water flea D. carinata, the EC50 value of 48h immobilization experiment was 96.3 µg/L for Cr. growth inhibition of the algae cells was determined following exposure for 72h and EC50 values of Cr was 386.1 µg/L. The results showed that Cr is highly toxic to D. carinata, and toxic to algae Scenedesmus sp. Based on the observed high sensitivity with Cr, both two organisms are potential bioindicators for the assessment of chromium pollution in Sài Gòn River. However, water flea D. carinata was more sensitive than freshwater algae Scenedesmus. On another hand, algae Scenedesmus sp. calls for further investigation on chromium uptake capacity and utilization in chromium contaminated water treatment.
INTRODUCTION
In Việt Nam, fresh surface water pollution and deterioration has been an important problem because of its impact on human health and aquatic ecosystem. In practical, detail and extensive monitoring of fresh water quality are limited due to the shortage of human and financial resources. In recent years, the quickly induction of urbanization and industrialization become main reasons of increasing water demands, and water pollution caused by anthropogenic activity. Therefore, it called for a better procedure to monitor the quality of water in Sài Gòn River system, which are important water resources for Hồ Chí Minh, the biggest city in Southern of Việt Nam. Among variety of contaminants which can pollute the fresh water and toxic to human health, heavy metals are great concern due to its toxicity and tolerance once introduced into the aquatic environment. The objective of this study was to investigate the possibility of using tropical fresh water living creatures to detect the contaminated Cr in the water through acute toxicity test. This study is going to contribute to provide a simple and cost effective method to quickly assess the toxicity of fresh water quality, to protect the water resource and health of water users.
Metals are among the most intensively studied pollutants in fresh water environments. Many of metals are important for live processes at very low concentrations, but at higher doses they become toxic (Warnau et al., 1995). Metals can be introduced into environment from many anthropogenic activities such as industrial, agricultural, and mining processes, then they become tolerant pollutants and pose significant risks on living creatures in the ecosystem including human (Lanctôt et al., 2016; Schwarzenbach et al., 2010; Tomasiks and Warren, 1996). While some metals play vital roles in living processes of organisms, some others do not. On another hand, it is worth to note all metals become toxicants while reach a concentration threshold (Wetzel, 2001). The previous studies have pointed out that metals are indestructible and can be accumulated in body of organisms (Lau et al., 1998; Waykar and Shinde, 2011), then transferred to higher trophic levels of the food chain (Ikemoto et al., 2008). The toxic effects of metals to living organisms have been well defined and considered as a major threat to aquatic biodiversity (Lanctôt et al., 2016; Millennium Ecosystem Assessment (Program), 2005; Moldovan et al., 2013; Van et al., 2013). Recently, it has been found that toxicity of dissolved metals in water is regulated by variety of water physical and chemical characteristics such as pH, alkalinity, dissolved organic carbon (DOC) and hardness (De Schamphelaere and Janssen, 2004; Hoang et al., 2004; Jo et al., 2010; Linbo et al., 2009; Ryan et al., 2009). Therefore, it is necessary to take the water samples of interested area, and use it to prepare the toxicity test using tropical organism to define the sensitivity level.
In this study, two tropical freshwater micro crustacean and phytoplankton species, including D. carinata and Scenedesmus sp., were screened in terms of sensitivity to Cr for cost effective
pollution monitoring. The two living organisms were chosen due to high sensitivity of micro crustacean Daphnia to dissolved heavy metals in water, while planktonic algae are easy to culture, and require only small laboratory space and simple equipment. Algae are primary producers of which population growth inhibition can be used as criterion of response in toxicity test. Moreover, the inhibition of algae’s population in aquatic environment can imply the chain reaction on ecological food chains in water environment.
The purpose of this study is to develop and optimize a procedure using a battery of organisms for use in routine monitoring of freshwater of Sài Gòn River. One of the first criteria for toxicity test is sensitivity of organism to contaminant of interest. Therefore, we aim to develop a practical process which enables to detect Cr pollution in fresh water using a battery of organisms. The test battery consists of two species representatives of two consecutive trophic levels: micro algae Scenedesmus sp. (primary producer) and Daphnia carinata (primary consumer).
MATERIALS AND METHODS
Water samples collection
Surface water was collected from the upstream of Sài Gòn River (Dầu Tiếng Freshwater Reservoir). The water sample was transferred to the Environmental Toxicology Laboratory, Institute for Environment and Resources in Hồ Chí Minh City, filtered through 0.45 μm syringe filter (Sartorius, Germany) and stored at 4°C prior to the tests.
Water samples characteristics
The filtered waters from Dầu Tiếng Reservoir was analyzed for water quality parameters that may affect the bioavailability of dissolved metals and the survival and growth of the two organism of test battery, alkalinity and hardness, pH, trace metals and pesticides. Total hardness was determined based on con- centration of Ca2 + and Mg2 +, metals were analysis by ICP/MS.
Test organisms
Organisms used in the present study were D. carinata and freshwater algae Scenedesmus sp. These species were collected from the field in Việt Nam and have been cultured in the Ecotoxicology Laboratory (Institute for Environment and Resources) for over a year. D. carinata were cultured in 1.2 L beakers with 1.0 L of COMBO medium (Kilham et al., 1998). The light intensity was approximately 1000 lux. The crustaceans were fed with a mixture of green alga (Chlorella sp.) and YCT (yeast, carophyl and trout chow digestion), prepared according to the US Environmental Protection Agency Method (US EPA, 2002) with a modification to the algal culture medium, which was the COMBO medium. Algae Scenedesmus sp. were culture in COMBO medium.
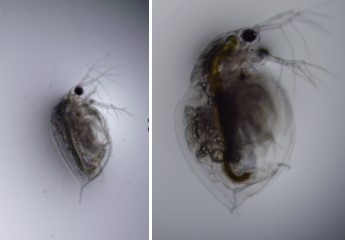
Morphology of Daphnia carinata (from left to right: male and female)

Fresh water algae Scenedesmus sp in culture flasks
Acute toxicity tests
The 48-h static nonrenewal acute toxicity tests were conducted following the guidelines of the US EPA methods (US EPA, 2002) with two adjustments of: i) light regime (a photoperiod of 12 h:12 h light dark at a light intensity of ca. 1000 Lux) and ii) temperature (27 ± 1°C) for tropical species. Neonates of D. carinata (age ≤ 24h) were used for testing. Each treatment had four replicates and each replicate consists of 10 neonates in 40 mL of exposure solution in a 50-mL polypropylene cup. The neonates were fed during the pre-exposure duration but starved during the tests (US EPA, 2002). Chromium treatments were prepared by spiking Cr in constituted medium prepared with field-collected water. K2Cr2O7 was used as Cr salt. Five concentrations of Cr were prepared for each metal exposure. Controls were prepared by transferring the neonates into the constituted medium without metal addition.
We checked daily for immobilized organisms and removed them from the cups. Immobilization data were used to determine median lethal concentrations (48 h-LC50). At the end of the test, test solution in one of the four replicates was randomly taken (in each metal concentration) for the metal analysis by ICP/MS.
Algal inhibition test
Bioassays were performed using the green algae Scenedesmus sp. To analyze the toxic effect of Cr on the algal growth, serial concentrations of K2Cr2O7 were tested using the COMBO media prepared without EDTA (Kilham et al., 1998). The initial inoculum cell density was 2±0.2 104 cells/mL and the assays were performed in triplicate using 125 mL flasks containing 25mL of medium. Cultures were incubated at 24°C in constant light (4.000 Lux) and the algal growth was estimated by absorbance readings at 750 nm after 96h incubation. The effective concentrations of metal inducing 50% effect (EC50) were calculated by plotting the values for the percent inhibition in average specific growth rate against the logarithmic value of the test substance concentration. Using the regression equation, determine the 50% inhibition concentration (EC50).
RESULTS AND DISCUSSION
The aim of this study is to develop a practical and cost effective procedure to detect chromium contamination in freshwater of Sài Gòn River. The two organisms were selected due to they originated from tropical freshwater environment and therefore easy to use in Việt Nam environmental condition.
In order to evaluate the applicable of the procedure in detection of Cr once introducing into freshwater of Sài Gòn River, field water sample was obtained and spiked with Cr at different concentration, then put in our toxicity test procedure with a battery of two organisms to detect Cr contamination. The chemical analyzes result of freshwater from upstream of Sài Gòn River showed good quality, without metals or herbicides contamination (Table 1).
Table 1: Dissolved metal concentrations (μg/L) and physical characteristics of filtered field water from Sài Gòn River used for the test. BDL, below detection limits of the ICP/MS. N/A, not available
|
Nr. |
Parameter |
Value |
Nr. |
Parameter |
Value |
|
1 |
TSS (mg/l) |
5 |
17 |
Cd (mg/l) |
BDL (LOD = 0.00004) |
|
2 |
Hardness (mg CaCO3/l) |
14 |
18 |
Pb (mg/l) |
0.0032 |
|
3 |
COD (mgO2/l) |
7 |
19 |
Cr (mg/l) |
0.006 |
|
4 |
N-NH4+ (mg/l) |
BDL (LOD = 0.03) |
20 |
Cu (mg/l) |
0.090 |
|
5 |
Cl- (mg/l) |
4,10 |
21 |
Ni (mg/l) |
BDL (LOD = 0.004) |
|
6 |
N-NO3- (mg/l) |
0.24 |
22 |
Mn (mg/l) |
0.010 |
|
7 |
P-PO43- (mg/l) |
BDL (LOD = 0.01) |
23 |
Hg (mg/l) |
BDL (LOD = 0.0003) |
|
8 |
Total N (mg/l) |
BDL (LOD = 1) |
24 |
Se (mg/l) |
BDL (LOD = 0.006) |
|
9 |
Total P (mg/l) |
0.02 |
25 |
Ag (mg/l) |
BDL (LOD = 0.003) |
|
10 |
SO42- (mg/l) |
2.17 |
26 |
Lindan (µg/l) |
BDL (LOD = 0.006) |
|
11 |
Al (mg/l) |
3.34 |
27 |
Aldrin (µg/l) |
BDL (LOD = 0.01) |
|
12 |
Ca (mg/l) |
9.91 |
28 |
Dieldrine (µg/l) |
BDL (LOD = 0.01) |
|
13 |
Mg (mg/l) |
1.73 |
29 |
Endosulfan (µg/l) |
BDL (LOD = 0.01) |
|
14 |
Na (mg/l) |
2.42 |
30 |
4,4'-DDT (µg/l) |
BDL (LOD = 0.01) |
|
15 |
K (mg/l) |
2.35 |
31 |
4,4'-DDE (µg/l) |
BDL (LOD = 0.01) |
|
16 |
As (mg/l) |
BDL (LOD = 0.0005) |
32 |
4,4'-DDD (µg/l) |
BDL (LOD = 0.01) |
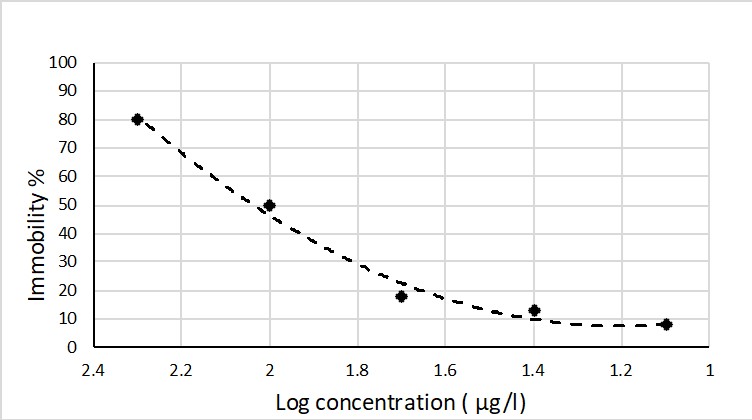
Figure 1: Daphnia concentration-immobilization rate curve. Log concentration of chromium is presented in x axis and immobility percentage of Daphnia at 48 hours is showed in y axis
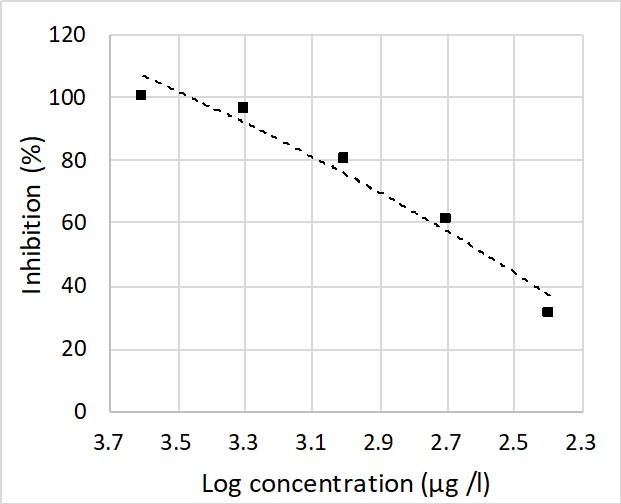
Figure 2: Algal concentration-inhibition (growth rate) curve. Log concentration of chromium is presented in x axis and growth rate inhibition percentage of Scenedesmus algae at 72 hours is showed in y axis
Chromium and chromium salts widely used in ore refining, chemical and refractory processing, cement-producing plants, automobile brake lining, catalytic converters for automobiles, leather tanneries.
Chromium is an essential micronutrient in carbohydrate metabolism cycle, but is toxic at high level. Hexavalent chromium is known to have 100-fold more toxicity than trivalent chromium, for both acute and chronic exposures because of its high-water solubility and mobility, as well as easy reduction. Therefore, this form of chromium is more strictly considered in water quality monitoring. At low level, chromium can cause harmful effect on aquatic organism. For instance, at 62 ppb it inhibits the growth of algae. In many case, aquatic animals are found more sensity to chromium than aquatic plants (Wright and Welbourn, 2002). Like other metals, chromium toxicity change according to temperature, pH, and salinity. Beside of that, chromium toxicity is higher in low hardness water environments.
Due to increasing of industrialization in recent year, it is the risk of Cr contamination of the Freshwater Reservoir and therefore it is required better supervision to detect Cr pollution in this freshwater body. For D. carinata, with the modified ISO test medium with pH 7.0, EC 168.8 (µS/Cm), DO = 6.8 (mg/L) and temperature 29oC, the EC50 value of 48h immobilization experiment was 96.3 µg/L. The result showed that the sensitivity of D. carinata in this study to copper was comparable with Daphnia magna with EC50 was 130 µg/L (Okamoto et al., 2015).
The results clearly demonstrated that the proposed toxicity testing procedure using D. carinata in this study has high sensitivity to detect Cr contamination in freshwater of Sài Gòn River. And therefore, can be considered as a useful tool for Cr pollution monitoring control in the Freshwater Reservoir. The EC50 value was 96.3 µg/l for Cd make this organism a promise tool for detection Cr pollution. According the Law on Environmental Protection of Việt Nam, the highest allowance level of Cr can be up to 1000 µg/l. Therefore, with EC50 value 96.3 µg/l the proposed testing procedure using D. carinata is sensitivity and easy to apply to detect chromium contamination.
Beside of D. carinata, freshwater microalgae Scenedesmus sp. was also use to detect chromium pollution. The combination use of different organisms could increase the reliability of heavy metal pollution detection. Using COMBO medium without EDTA prepared with water collected from Dầu Tiếng Reservoir. The test medium has pH = 7.6, EC = 278 (µS/cm), DO = 6.9 (mg/L) and temperature 26oC. The result pointed out the growth inhibition effect of Cr on the fresh water algae. The IC50 value was 386.1 µg/L was lower than Scenedesmus obliquus (620 µg/L) which has been reported previosly (Pavel and Cepák, 2005).
In Việt Nam, the highest allowance value of Cr in surface water is 1000 µg/L. Therefore, the EC50 value of S. cenedesmus in this study was lower than the legal threshold of water quality and can be a potential tool to detect Cr contamination water samples.
CONCLUSION
The result showed that both two organisms are potential bio-indicators for the assessment of chromium pollution in Sài Gòn River. However, water flea D. carinata was more sensitive than freshwater algae Scenedesmus. Moreover, the algae Scenedesmus may be useful in bioremediation of Cr contamination waste water by simply adding required nutrients to support algae growth and they may extract Cr from water into its biomass. Of course, this potential strategy need further study to investigate the ability to absord and accumulate Cr in cells of the fresh water algae. The cost effective and practical proposed toxicity test procedure in this study can be a useful tool to monitor water quality and detect Cr contamination in Sài Gòn River as the first protection barrier. Then, more detail chemical analysis can be combined later to precisely identify inspect toxic pollutants.
Acknowledgments: This research is funded by VNU of Hồ Chi Minh city (VNU-HCM) under grant number B2018-24-02 was acknowledged.
REFERENCE
1. De Schamphelaere, K.A.C., Janssen, C.R., 2004. Effects of chronic dietary copper exposure on growth and reproduction of Daphnia magna. Environ. Toxicol. Chem. 23, 2038 - 2047.
Hoang, T.C., Tomasso, J.R., Klaine, S.J., 2004. Influence of water quality and age on nickel toxicity to fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 23, 86–92.
Ikemoto, T., Tu, N.P.C., Okuda, N., Iwata, A., Omori, K., Tanabe, S., Tuyen, B.C., Takeuchi, I., 2008. Biomagnification of trace elements in the aquatic food web in the Mekong Delta, South Vietnam using stable carbon and nitrogen isotope analysis. Arch. Environ. Contam. Toxicol. 54, 504–515.
Jo, H.-J., Son, J., Cho, K., Jung, J., 2010. Combined effects of water quality parameters on mixture toxicity of copper and chromium toward Daphnia magna. Chemosphere 81, 1301–1307.
Kilham, S.S., Kreeger, D.A., Lynn, S.G., Goulden, C.E., Herrera, L., 1998. © 1998 Kluwer Academic Publishers. Printed in Belgium. 147 COMBO: a defined freshwater culture medium for algae and zooplankton.
Lanctôt, C., Wilson, S.P., Fabbro, L., Leusch, F.D.L., Melvin, S.D., 2016. Comparative sensitivity of aquatic invertebrate and vertebrate species to wastewater from an operational coal mine in central Queensland, Australia. Ecotoxicology and Environmental Safety 129, 1–9.
Lau, S., Mohamed, M., Tan Chi Yen, A., Su’ut, S., 1998. Accumulation of heavy metals in freshwater molluscs. Science of The Total Environment 214, 113–121.
Linbo, T.L., Baldwin, D.H., McIntyre, J.K., Scholz, N.L., 2009. Effects of water hardness, alkalinity, and dissolved organic carbon on the toxicity of copper to the lateral line of developing fish. Environ. Toxicol. Chem. 28, 1455–1461.
Millennium Ecosystem Assessment (Program) (Ed.), 2005. Ecosystems and human well-being: synthesis. Island Press, Washington, DC.
Moldovan, O.T., Meleg, I.N., Levei, E., Terente, M., 2013. A simple method for assessing biotic indicators and predicting biodiversity in the hyporheic zone of a river polluted with metals. Ecological Indicators 24, 412–420.
Okamoto, A., Yamamuro, M., Tatarazako, N., 2015. Acute toxicity of 50 metals to Daphnia magna: Acute toxicity of 50 metals to D. magna. J. Appl. Toxicol. 35, 824–830.
Pavel, P., Cepák, V., 2005. Chromium influences growth and cell morphology but itself does not induce gametogenesis in three Scenedesmus obliquus strains. Fottea 5, 91–100.
Ryan, A.C., Tomasso, J.R., Klaine, S.J., 2009. Influence of pH, hardness, dissolved organic carbon concentration, and dissolved organic matter source on the acute toxicity of copper to Daphnia magna in soft waters: implications for the biotic ligand model. Environ. Toxicol. Chem. 28, 1663–1670.
Schwarzenbach, R.P., Egli, T., Hofstetter, T.B., von Gunten, U., Wehrli, B., 2010. Global Water Pollution and Human Health. Annual Review of Environment and Resources 35, 109–136.
Tomasiks, P., Warren, D.M., 1996. The use of Daphnia in studies of metal pollution of aquatic systems. Environ. Rev. 4, 25–64.
Van, K.D., Janssens, L., Debecker, S., Jonge, M.D., Lambret, P., Nilsson‐Örtman, V., Bervoets, L., Stoks, R., 2013. Susceptibility to a metal under global warming is shaped by thermal adaptation along a latitudinal gradient. Global Change Biology 19, 2625 - 2633.
Warnau, M., Ledent, G., Temara, A., Jangoux, M., Dubois, P., 1995. Experimental cadmium contamination of the echinoid Paracentrotus lividus: influence of exposure mode and distribution of the metal in the organism. Marine Ecology Progress Series 116, 117–124.
Waykar, B., Shinde, S.M., 2011. Assessment of the Metal Bioaccumulation in Three Species of Freshwater Bivalves. Bull Environ Contam Toxicol 87, 267–271.
Wetzel, R.G., 2001. Limnology: Lake and River Ecosystems, 3 edition. ed. Academic Press, San Diego.
Wright, D.A., Welbourn, P., 2002. Environmental Toxicology, 1st ed. Cambridge University Press.
Đinh Hoàng Đăng Khoa1, Phạm Thị Thu Hằng1, Lê Hiền Minh Tâm 1, Trần Thị Yến Nhi1, Lê Phi Nga2
1Environmental Biotechnology Laboratory, Institute for Environment and Resources, Vietnam
National University (VNU)
2Department of Biotechnology, Hồ Chí Minh City University of Technology, VNU
(Nguồn: Bài đăng trên Tạp chí Môi trường số Chuyên đề Tiếng Anh III/2020)