
22/05/2025
Abstract:
The abundant coffee by-products in Vietnam have great potential for producing environmentally friendly adsorbent materials, increasing the value of processed coffee products, contributing to climate change mitigation. The objective of this study is to evaluate the microstructure of coffee husk biochar (BC) and potassium carbonate-activated biochar (BC-K2CO3) revealed the material has rough surface with numerous pores ranging from 100 nm to 300 nm. When used at a dosage of 0.1 g/mL with an adsorption time of 120 minutes and an initial caffeine concentration below 0.025 g/mL, it achieved an adsorption efficiency of over 89.75%. The kinetic caffeine adsorption of BC- K2CO3 aligns well with the Freundlich adsorption isotherm model (R² >0.992). BC-K2CO3 exhibited a maximum adsorption capacity (Qm = 33.74 mg/g), significantly higher than the control sample biochar BC (Qm = 13.99 mg/g), which was produced under the same pyrolysis conditions without K2CO3 activation
Keywords: Biochar, coffee husk, biochar surface area, maximum caffeine adsorption capacity.
JEL Classification: Q56,Q57,Y10, O13,R11.
Received: 2nd January 2025; Revised: 7th Febuary 2025; Accepted: 2nd March 2025.
1. INTRODUCTION
Vietnam is the world's leading exporter of Robusta coffee, significantly contributing to the national economy. The coffee processing industry generates coffee husks at a rate of 0.6 - 0.78 kg of husk per 1 kg of green coffee beans. This husk can be converted into valuable materials, such as ammonium adsorbents, nutrients, fertilizers, cyclic chemicals, inks, aromatic substances, pharmaceuticals (notably caffeine), and adsorbents for caffeine removal from aqueous environments, caffeine-containing extracts, or wastewater. Additionally, there is an increasing demand for safe and effective caffeine adsorbents to produce caffeine-free coffee products, especially for individuals sensitive to caffeine[1-18].
The use of water and activated carbon adsorbents for caffeine removal in decaffeinated coffee production (decaf coffee beans) provides an alternative to traditional methods that rely on environmentally harmful organic solvents. While activated carbon has been widely used for this purpose, the activation of biochar derived from coffee husks in this study presents an opportunity to develop caffeine adsorbents for water treatment as well as for producing high-value caffeine-free coffee extracts. Replacing conventional caffeine removal methods which often use hazardous chemical solvents such as methylene chloride, chloroform, or the less toxic ethyl acetate helps address environmental concerns, chemical residues, and cost issues[1].
Supercritical CO2 extraction, proposed by Zosel[2], is a more environmentally friendly approach but requires high equipment costs due to the need for high pressure. The use of biochar has emerged as a sustainable and promising solution[3]. Studies such as those by Elvio et al. (2021) have demonstrated the significant caffeine adsorption capacity of activated carbon derived from coconut leaves[4]. Similarly, Keerthanan et al. (2020) highlighted the advantages of biochar derived from pyrolyzed tea plant residues[5].
Our research focuses on utilizing biochar derived from coffee husks activated with K2CO3 (BC- K2CO3) and comparing it with non-activated biochar (BC). The objective is to develop an environmentally friendly and cost-effective caffeine removal material and method that promotes waste recycling without the use of harmful chemicals[3]. This approach aims to repurpose coffee husks for the selective adsorption of caffeine, contributing to the production of value-added materials from coffee by-products and high-value decaffeinated coffee products. The outcome of this study is the development of a novel, eco-friendly biochar material for caffeine adsorption, offering environmental, economic, and public health benefits while supporting the production of decaffeinated coffee beans.
2. MATERIALS AND METHODS
2.1. Material
Coffee husk and green coffee bean were collected from Cư M’gar - Dak Lak (Dak Lak – Vietnam).
2.2. Experiments
Preparation of biochar samples
Method 1: Making biochar of coffee husks (BC)
3 grams of green coffee husks were tightly packed into a cup with a lid. Slow pyrolysis was conducted under anaerobic conditions within the cup. The temperature was gradually increased to 400 degrees Celsius and maintained for 30 minutes. This process resulted in the production of BC biochar.
Method 2: Making biochar activated with K2CO3 (BC- K2CO3)
BC and K2CO3 were mixed at a 1:3 mass ratio and allowed to react for 2 hours. Afterward, the reacted sample was tightly packed into a cup with a lid. Slow pyrolysis was then conducted under anaerobic conditions. The temperature was gradually increased at a rate of 10 degrees Celsius per minute until it reached 400 degrees Celsius. This temperature was maintained for 30 minutes. This process resulted in the production of BC-K2CO3 biochar.
Method 3: Making caffeine extraction of green coffee beans
The coffee bean was grounded and impregnated with deion water at the rate of 10 mL.g-1 with a heating of 80ºC for 3 hours. Coffee bean extract was determined at pH = 6.7 and stored at -4ºC for further experiments.
Setting up experiments
The influence of contacting time (10-180 minutes) on the treatment efficiency was carried out at the pH of 6.7; biochar dose of 0.05 g.mL-1, agitation of 100 rpm. The influence of the biochar dose (0.01 - 0.15 g.mL-1) on the caffeine removal capacity was carried out at pH of6.7, agitation of 100 rpm for 120 minutes. The influence of initial caffeine concentration on treatment efficiency was carried out at pH of6.7, biochar dose was 0.15 g.mL-1, agitation of 100 rpm for 120 minutes.
Analysis methods: The biochar microstructure was analyzed by SEM technique, The FTIR was done by Bruker Tensor 27 IR (USA) in spectral range from 400 cm-1 to 4000 cm-1.[6]
Adsorption isotherm and kinetic model
The caffeine adsorption isotherm of biochar was evaluated by Langmuir (1) and Freundlich (2) models.[7]

where: qe (mg.g-1) and Ce (mg.l-1) are equilibrium ammonium ion concentrations in solid phase and liquid phase, respectively; Qm (mg.g-1) is the maximum adsorption capacity of the material and b (kl.g-1) is the equilibrium constant related to the adsorption energy; KF, n are Freundlich constants. The adsorption rate is first-order (3), or second-order (4), dependent on the capacity of the adsorbent.[8]

where: Qt (mg.g−1) is adsorption capacity at time t (min), Qe (mg.g−1) is adsorption capacity at equilibrium time, k1 (min−1) and k2 (g.mg−1.min−1) are rate constants.
3. RESULTS AND DISCUSSION
3.1. Characteristics of biochar
The SEM imaging presented in Figure 1 and 2 show that the material has a rough surface, many pores with pore size in the range of 100 nm to 300 nm (BC-K2CO3). This feature contributes to the material's good adsorption and retention capacity for pollutants. Thermal treatment at elevated temperatures can induce the decomposition of constituent components such as cellulose and lignin within the material, concurrently leading to an expansion of its pore structure. This phenomenon is associated with an enhanced adsorption efficiency.[8,9,10]


Figure1: SEM images of BC
The FTIR spectra (Figure 3 and 4) showed that the functional groups on the surface of the biochars are in the range of 500-4000 cm-1. The wide spectral band in the range of 3500-3000 cm-1 of both biochar due to the OH group being stretched and the OH group present in cellulose, lignin, water or can also correspond to the N-H valence oscillation in the amine (first-order and second-order) and carbohydrate groups in macromolecular compounds; the spectral band near the 1500 cm-1 value indicates that the C=C bond is stretched in the structure of both biochars; spectral in the range of 1300 - 1000 cm-1 shows the appearance of C-O bond.[11]


Figure2: SEM images of BC- K2CO3

Figure 3. FTIR spectra of BC-K2CO3

Figure 4. FTIR spectra of BC-K2CO3 after caffeine adsorption
3.2. Effect of contacting time on the adsorption
The effect of contacting time on caffeine treatment efficiency was carried out in the time from 10 to 180 min at initial pH 6.7. The Figure 5 resulted that after the first 30 minutes, the adsorption rate increased slowly. Between 30 and 120 min, the adsorption rate continued to increase, then gradually stabilized and reached equilibrium after 120 min in both BC and BC-K2CO3, with the treatment efficiency reaching 39.48% and 62.01%. This trend could be explained as follows: the initial rapid adsorption is due to the caffeine replacing the positive ion on the material's surface. In the first time of contact, the biochar has a lot of vacant adsorption sites, the caffeine concentration in the water is the highest, so the adsorption process is high and leads to a rapid increase in the process efficiency. The next slower rising phase represents physical adsorption deterioration with ionic equilibrium. The slow increase is due to chemisorption and diffusion of caffeine inside the material, then reaches saturation of the active sites.[12]

Figure 5. Effect of contact time on caffeine removal efficiency byBC and BC-K2CO3
3.3. Effect of biochar on the adsorption capacity
The biochar dosage (g.mL-1) was carried out at pH 6.7, contact time was 120 min, there was a change in the ratio between the mass of the material and the volume of the solution in the range from 0.01 to 0.15 g.mL-1. Figure 6a and 6b showed that the caffeine adsorption efficiency increased rapidly from 22.89% to 44.55% for BC and from 31.29% to 53.36% for BC-K2CO3 when the biochar dosage was in the range from 0.01 to 0.1 g.mL-1.
For BC and BC-K2CO3, when the biochar dosage increased to 0.15 g.mL-1, the treatment efficiency did not increase too much and the biochar dosage for the highest adsorption efficiency is 0.15g.mL-1. When the dosage is higher, the contact surface will be larger and more caffeine will be adsorbed on the surface. However, when the biochar dosage was increased to a certain value, the caffeine adsorption was not significantly increased because the overlap of adsorbent layers could obscure the active sites.

Figure 6a. Biochar dosage-caffeine removal efficiency and adsorbed on the surface of BC-K2CO3

Figure 6b. Biochar dosage-caffeine removal efficiency and adsorbed on the surface of BC
3.4. Effect of initial caffeine concentration on the adsorption capacity
To investigate the influence of initial caffeine concentration, experiments were carried out with BC and BC-K2CO3 materials at biochar dosage of 0.05 g.mL-1, agitation of 100 rpm for 120 min. The initial caffeine concentration was investigated ranging from 0.02 to 0.27 mg.mL-1. Figure 7 showed that the initial caffeine concentration was 0.02 mg.mL-1, the treatment efficiencies of BC and BC-K2CO3 were 75.1% and 89.7%, respectively. With an increase in initial caffeine concentration from 0.02 to 0.27 mg.mL-1, the efficiency decreased from 75.1% to 30.3% with BC and from 89.7% to 41.6% with BC- K2CO3. This reduction can be attributed to the limited maximum adsorption capacity of the materials.[13] When the material surface does not have enough adsorbent sites to adsorb caffeine, increasing the concentration of caffeine solution while keeping the same dose of material will cause the amount of free caffeine to increase while the adsorbed caffeine remains unchanged, leading to the adsorption efficiency was gradually reduced.
Caffeine adsorption isotherm: The studies of equilibrium in adsorption indicate the biochar adsorption capacity by Langmuir and Freundlich models that have been widely used. The experimental results are shown in Figure 8 and 9, and the constants are shown in Table 1. The suitability between the model and experimental data is shown by the correlation coefficient R2. The correlation coefficient R2 in Table 1 shown that caffeine adsorb with biochar is more consistent with Freundlich adsorption theory for both materials BC and BC-K2CO3 (R2 of 0.992 and 0.963).

Figure 7. Effect of initial caffeine concentration on caffeine adsorption efficiency by BC and BC-K2CO3
For the Langmuir theory, the R2 values of the two materials are both in the range of 0.8 to 0.9. It could be seen that the adsorption of biochar can not only describe the linear or saturation region and the working concentration range. The caffeine adsorbant follows both monolayer and multilayer mechanisms. The caffeine extraction process may not follow a single adsorption mechanism, but rather multiple mechanisms (ion exchange, chemisorption, complexation...). Therefore, the use of the Langmuir or Freundlich adsorption theory to describe the adsorption process by the experimental material can only provide an approximate description.
Table 1: Parameters of isotherm model and R2
|
Parameters |
Parameter values |
R2 |
||
|
BC |
BC-K2CO3 |
BC |
BC-K2CO3 |
|
|
Langmuir |
|
|
0.864 |
0.806 |
|
b (L.mg-1) |
0.029 |
0.015 |
||
|
Qm (mg.g-1) |
13.99 |
33.74 |
||
|
Freundlich |
|
|
0.992 |
0.963 |
|
K (mg.g-1) |
1.199 |
1.197 |
||
|
n |
0.834 |
0.692 |
||
Caffeine adsorption kinetic: Results have been shown in Table 2 and Figure 10 and 11. Table 2 showed that the correlation coefficient R2 for each material in both pseudo-second-order kinetic models is very high (greater than 0.999). For the BC-K2CO3 material, the R2 value for the second-order model reaches a value of 1. However, the experimental qe value also needs to be compared with the qe value calculated from the two models. The second-order model gives qe value closer to the experimental value. Therefore, it can be concluded that the caffeine treatment process fits the pseudo-second-order kinetic model.
Table 2. Kinetic models, parameters and R2
|
Parameters |
Parameters |
R2 |
||
|
BC |
BC- K2CO3 |
BC |
BC- K2CO3 |
|
|
Pseudo-first order |
|
|
0.934 |
0.773 |
|
k1 (min-1) |
0.038 |
0.040 |
||
|
qe1 (mg.g-1) |
1.48 |
1.94 |
||
|
Pseudo-second order |
|
|
0.999 |
1 |
|
k2 g.(mg.min)-1 |
0.624 |
1.758 |
||
|
qe2 (mg.g-1) |
1.48 |
1.94 |
||

Figure 8. Langmuir model
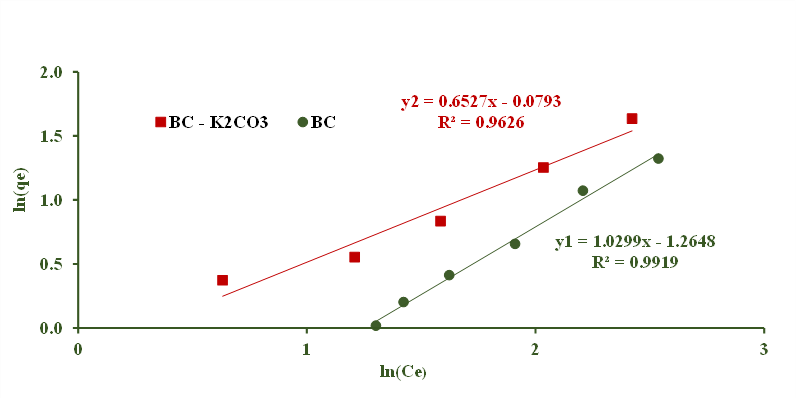
Figure 9. Freundlich model

Figure 10. Pseudo-first-order kinetic model

Figure 11. Pseudo-second-order kinetic model
3.5. Comparison of caffeine adsorption by various adsorbents
Previous studies have investigated the adsorption of caffeine by various adsorbents, including activated carbon, carbon xerogel, and biochars. Activated carbon and carbon xerogel exhibited high caffeine adsorption capacities due to their extensive surface areas.[14,15] However, their production often involves significant use of chemicals and high energy consumption, leading to increased costs. Consequently, there is growing interest in utilizing environmentally friendly waste materials as low-cost alternatives for contaminant remediation.While fique bagasse biochar displayed a caffeine adsorption capacity of 3.52 – 9.13 mg.g⁻¹,[16] and woodchip biochar a capacity of 13.2 mg.L⁻¹,[17] this study presents a novel approachof using coffee husk activated by K2CO3 at suitable temparature 4000C that could be easily made by local biochar maker equipment at local site. We engineered BC-K2CO3 and make the comparison with previously reported, our BC-K2CO3 coffee husk biochar activated by K2CO3 adsorbents caffein with high adsorption capacity Qm = 33.74 mg.g-1.
4. CONCLUSION
The abundant coffee by-products in Vietnam have great potential for producing environmentally friendly adsorbent materials, increasing the value of processed coffee products, contributing to climate change mitigation. BC-K₂CO₃-activated coffee husk biochar features a rough surface with numerous pores ranging from 100 nm to 300 nm. When used at a dosage of 0.1 g/mL with an adsorption time of 120 minutes and an initial caffeine concentration below 0.025 g/mL, it achieved an adsorption efficiency of over 89.75%. The kinetic caffeine adsorption of BC-K₂CO₃ aligns well with the Freundlich adsorption isotherm model, with a high correlation coefficient R² (>0.992). BC-K₂CO₃ exhibited a maximum adsorption capacity (Qₘ = 33.74 mg/g), significantly higher than the control sample biochar BC (Qₘ = 13.99 mg/g), which was produced under the same pyrolysis conditions without K₂CO₃ activation. This study opens a new development direction for caffeine adsorbent materials derived from coffee husks, pruned branches, and spent coffee grounds, enabling the creation of high-value products and promoting a circular economy. In the future, further research is needed to optimize biochar production (including expansion of biomass sources, pyrolysis conditions, and activation methods) to create green materials with selective adsorption and improved efficiency. In particular, supportive policies are needed to encourage research on green products, promote their application and commercialization, and assist businesses and producers in implementing circular economy models. Such initiatives, based on the reuse of by-products from coffee production and food processing, will benefit the environment and public health while adding value and enhancing the reputation of Vietnam’s manufacturing sectors.
Acknowledgments. The study was funded by the Vietnam Academy of Science and Technology for the Senior Researcher Program (code NCVCC07.08/24-24) on coffee husk biochar for caffeine adsorption. Additional support came from a project on decaffeination technology using biochar to recirculate extract solutions and reuse waste at small-scale coffee production facilities funded by the People’s Committee of Đắk Lắk Province.
Ngô Kim Chi 1*, Nguyễn Hoài Linh1 , Đặng Ngọc Phượng1, Phạm Phương Thảo2, Trần Lê Minh2,
Đỗ Thủy Tiên3, Vũ Phương Uyên3, Đặng Văn Hưng4, Hoàng Thgij Thu Hương2
1Vietnam Academy of Science and Technology (VAST), 2Hanoi University of Science and Technology,
3 Hanoi Pedagogical University,
4 University of Science - Vietnam National University
(Source: The article was published on the Environment Magazine by English No. I/2025)
REFERENCES
A. Vandeponseele, M. Draye, C.Piot, G.Chatel. Study ofInfluential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions, Clean Technologies, 2021, 3, 335–350.
K. Ramalakshmi. Caffeine in Coffee: Its Removal. Why and How?, Critical Reviews in Food Science and Nutrition, 1999, 39, 441-56.
C. Donyau, Y.L.Chih, Chen, T.H. L. Sanboh. Caffeine Extraction from Raw and Roasted Coffee Beans, Journal of Food Science, 2018,83(4), 975-983.
N. Elvio, T. Alex. Highly effective adsorption of caffeine by a novel activated carbon prepared from coconut leaf, Environmental Science and Pollution Research, 2022, 29, 50661–50674.
S. Keerthanan, B. Amit, M. Kúhani, J. Chamila. Engineered tea-waste biochar for the removal of caffeine, a model compound in pharmaceuticals and personal care products (PPCPs), from aqueous media, Environmental Technology & Innovation, 2020, 19, 100847.
H. Xiaojian, Z. Xinbo, H. H. Ngo, G.Wheshan, W. Haitao, L. Chaocan, Z. Yongchao, M. Chạnuan. Comparison study on the caffeine adsorption of the biochar derived from different kinds of fruit peel, Science Total Environment, 2020, 707, 135544.
B. Amit, A. K. Minocha. Biosorption optimization of nickel removal from water using Punica granatum peel waste, Colloids Surfaces B Bio interfaces, 2010, 76, 544–548.
E. V. Antonakou, L. Angelous, H. N. Merete, B. Aud. Evaluation of various types of Al-MCM-41 materials as catalysts in biomass pyrolysis for the production of bio-fuels and chemicals, Fuel, 2006, 85,2202-2212.
T.B.V. Dhyani. A comprehensive review on the pyrolysis of lignocellulosic biomass, Renew. Energy, 2018, 129, 695–716.
C.Liang, G.Gascó ,F. Shenglei, M.Ana. Biochar from pruning residues as a soil amendment: Effects of pyrolysis temperature and particle size, Soil and Tillage Research, 2016, 164, 3-10.
S.Biswajit, K.Tarun, K.B.Ashim, D. Sudip. Removal of Cr (VI) from aqueous solution using natural plant material, Journal of Applied Sciences in Environmental Sanitation, 2007, 2 (3), 77-83.
D.Kucić, I.Cosić, M.Vuković, F. Briski. Sorption kinetic studies of caffeine from aqueous solution on different inorganic and organic media, Acta Chimica Slovenica, 2013, 60, 109- 119.
C.Manisha, K.Rahul, N.Sudarsan. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water, Journal of Hazardous Materials, 2020, 392, 122441.
J.L.Sotelo, G. Ovejero, A. Rodríguez, S. Álvarez, J. Galán, J.García. Competitive adsorption studies of caffeine and diclofenac aqueous solutions by activated carbon, Chemical Engineering Journal, 2014, 240, 443–453.
Ptaszkowska-Koniarz, M. Goscianska, J. Pietrzak. Synthesis of carbon xerogels modified with amine groups and copper for efficient adsorption of caffeine Chemical Engineering Journal, 2018,345, 13–21.
Correa-Navarro, Y.M. Moreno-Piraján, J.C. Giraldo, L. Rodríguez-Estupiñan. Caffeine adsorption by fique bagasse biochar produced at variouspyrolysis temperatures,Oriental Journal of Chemistry, 2019, 35, 538–546.
O.Muter, I. Perkons, V. Bartkevičs. Removal of pharmaceutical residues from wastewater by woodchip-derived biochar, Desalination and Water Treatment, 2019, 159, 110–120.